Abstract
Background: Asparaginase (ASNase) has become a key component of treatment for acute lymphoblastic leukemia (ALL). The ability of this agent to eliminate leukemic blasts by utilizing the special feature that such cells are largely unable to synthesize the otherwise non-essential amino acid asparagine means that blasts cannot survive in an environment in which this amino acid has been depleted.
Currently, there are three ASNase products commercially available sharing the same mechanism of action. The oldest one is the purified native enzyme extracted from E. coli. This product was further developed and refined by conjugation with polyethylene glycol (PEG-L-Asparaginase, PEG-ASNase) to prolong half-life and to reduce immunogenicity. A third ASNase product derived from the bacterium Erwinia chrysanthemi (Erwinase) is structurally different from the E. coli ASNase products.
The main reason for limitation in the use of ASNase is the rather frequent occurrence of hypersensitivity reactions (HSR). Such reactions require the replacement of the ASNase product used with alternative preparations. The incidence of clinical HSR to ASNase varies with the type of ASNase product, the treatment schedule (number of ASNase doses, extended ASNase-free intervals) and the administration route (intravenously [IV] or intramuscularly [IM]).
This retrospective study was primarily performed to demonstrate the dynamics and the interdependence when using ASNase in a complex multiagent treatment protocol. It aimed to evaluate the incidence of clinically apparent HSR in the ALL-BFM 2000 trial which was part of the cooperative trial AIEOP-BFM ALL 2000.
Methods: Clinical hypersensitivity reactions (HSR) to native E. coli ASNase were retrospectively analyzed in a cohort of children with acute lymphoblastic leukemia (ALL) focusing on the impact of concurrent chemotherapy schedules in the risk-adapted and randomized treatment protocol and evaluating the prognostic relevance of asparaginase discontinuation.
Patients: Patients with ALL aged 1-18 years were treated risk-stratified with standard-risk (SR), medium-risk (MR) and high-risk (HR) regimens in trial ALL-BFM 2000. Schedule of intravenously administered native E. coli ASNase varied by risk group and randomization arm. Randomizations compared the glucocorticoids prednisolone and dexamethasone in induction and different reintensification regimens (Möricke A et al, BLOOD 127(17): 2101-2112 (2016)). In this trial, native E. coli ASNase was administered IV as first-line product during the induction and reinduction phases. In the high-risk arm, the phase of intensified consolidation included additional E. coli ASNase doses. PEG-ASNase (IV) or Erwinase (IM) were used as second- or third-line products after HSR occurring with native E. coli ASNase or the respective second-line product.
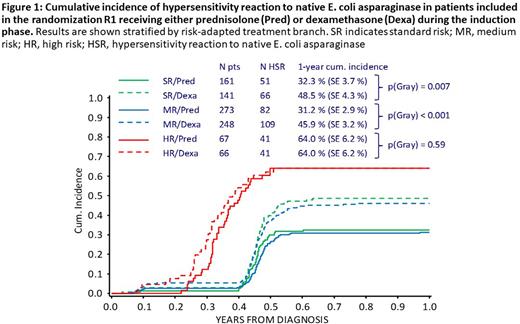
Results: HSR occurred in 40% (492/1207) of all evaluable patients. One-year cumulative incidence (CI-HSR±standard error) was 42.3±1.5% (40.6±2.5% in SR, 37.0±1.9% in MR, 65.2±3.8% in HR). Dexamethasone-treated SR and MR patients had significantly higher CI-HSR (48.5±4.3% and 45.9±3.2%) than prednisolone-treated patients (32.3±3.7% [p=0.007] and 31.2±2.9% [p<0.001]); no such difference was seen among HR patients who underwent multiple exposures to the drug (Fig. 1). Post-induction randomizations showed that one week of dexamethasone before asparaginase re-exposition in reinduction did not protect from clinical HSR in the SR/MR arm. Randomization in HR revealed an effect of the type of concurrent chemotherapeutic drugs on the risk of HSR. Discontinuation of ASNase occurred in 6.1% of all patients. No disadvantage in leukemia outcome could be detected for these patients.
Conclusion: The results in our study demonstrate the complex interdependencies contributing to the risk of HSR to E. coli asparaginase in a multiagent chemotherapy protocol. The differential effects of dexamethasone and prednisolone in induction generated new hypotheses about the role of glucocorticoids in sensitization or achievement of immune tolerance to asparaginase.
Disclosures
Möricke:Clinigen: Consultancy; BTG: Consultancy. Schrappe:JazzPharma: Consultancy, Honoraria, Research Funding; Servier: Honoraria, Research Funding; Amgen: Research Funding; Novartis: Honoraria, Research Funding; SHIRE: Research Funding; SigmaTau: Research Funding. Rizzari:Agmen: Honoraria; Servier: Honoraria; Jazz: Honoraria. Attarbaschi:Pfizer: Honoraria; MSD: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Jazz: Honoraria; Takeda: Honoraria; Gilead: Honoraria. Biondi:Amgen: Honoraria; Bluebird: Other: Advisory Board; Novartis: Honoraria; Incyte: Consultancy, Other: Advisory Board. Boos:Servier: Research Funding; Jazz: Research Funding. Conter:Medac: Research Funding; SigmaTau: Honoraria, Research Funding; Shire: Honoraria, Research Funding. Kulozik:Vertex: Honoraria; BMS: Honoraria; bluebird bio, Inc.: Honoraria, Research Funding; Celgene: Honoraria. Lanvers-Kaminsky:Servier: Other: travel grants; Clinigen: Honoraria. von Stackelberg:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Speakers Bureau; Clinigen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal